Transient ischemic dilatation (TID) of the left ventricle is a potential marker of high risk obstructive coronary artery disease on stress myocardial perfusion imaging (MPI). There is, however, interstudy variation in the diagnostic performance of TID for identification of severe and extensive coronary disease anatomy and varied prognostic implications.
There is increased cardiac event rates in patients with TID and an abnormal MPI. In otherwise normal perfusion, TID is an indicator of poor prognosis in patients with diabetes and/or a history of coronary disease.
67 years old gentleman asymptomatic underwent TMT for the insurance purpose and found to be abnormal and hence referred for stress myocardial perfusion imaging (stress MPI).
History of Diabetes Mellitus (23 years), HTN (31 years), Lipidemia (10 years)
Mild stress induced ischemic L.V. DILATATION.
Mild reversible perfusion abnormality in apex, minimal reversible perfusion abnormality in septal wall close to apex, inferior wall close to apex and inferio-lateral wall of the myocardium (mid to base) of the myocardium at stress (fig 1,2)
The above mentioned perfusion abnormalities are not appreciated quantitatively and hence statistically NOT significant (fig 3).
Predomainently mild stress induced ischemic l.V. Dilatation with insignificant stress induced myocardial ischemia quantitatively.
Subsequently patient underwent Coronary Angiography
Triple vessel disease (left main diffuse 40% disease), LAD proximal to mid diffuse 90% calcified disease, D1 ostial 70% disease, D2 ostial 70% disease followed by plaquing, LCX ostial 40% plaquing, distal plaquing, OM! Mid 50 to 60% disease, OM2 plaquing, RCA proximal 60%, distal 70% disease, PDA/PLB plaquing.
The apparent dilatation of the left ventricular cavity on MPI in the presence of extensive epicardial vessel stenoses has been referred to as transient ischemic dilatation. The mechanism of this apparent dilatation may involve subendocardial ischemia by revealing increased wall thickens on the delayed images, when cavity dilatation with myocardial thinning was seen during stress. The severely ischemic, count poor sub endocardium at stress appears as part of the left ventricular cavity with an external rim of slightly better perfused epicardium. The result is left ventricular cavity dilatation during stress MPI has become a generally accepted marker of severe, extensive myocardial ischemia. There are other causes of global subendocardial ischemia with apparent ischemic dilation of the left ventricle in the absence of any significant epicardial coronary artery stenoses.
Studies suggests severe hypertensive heart disease is an additional cause of global subendocardial ischemia resulting in transient ischemic dilatation of the left ventricular cavity seen on MPI.
The development of diffuse subendocardial ischemia with stress in hypertensive patients is likely caused by a combination of factors: namely, delayed diastolic left ventricular relaxation, significant endothelial dysfunction in the coronary arteries, decreased capillary density in hypertrophied myocardium, and elevated end-diastolic pressure in the left ventricle. The latter increases the epicardial diastolic pressure required to perfuse the full thickness of the myocardium.
Although in cases of severe left ventricular hypertrophy, the epicardial coronary arteries may show compensatory dilatation, as the hypertensive heart disease progresses, there appears to be patchy transmural ischemia in the myocardium, in addition to subendocardial ischemia, unassociated with any epicardial stenoses.
In severe multivessel coronary atherosclerosis, a similar combination of elevated left ventricular end-diastolic pressure and inadequate diastolic epicardial perfusion pressure exists. In this instance, end-diastolic pressures are elevated because of ischemia-induced systolic and diastolic myocardial dysfunction. The inadequate diastolic epicardial perfusion pressure is a result of multiple severe epicardial stenoses. Redistribution of blood flow away from the sub endocardium in the presence of coronary stenosis has been documented. A severe reduction in epicardial diastolic perfusion pressure creates a trans myocardial perfusion gradient that is visible on 201Tl myocardial perfusion imaging. This marked decrease in subendocardial perfusion during stress results in minimal uptake of radionuclide and, thus, an apparent dilatation of the left ventricular cavity on the stress images.
Transient ischemic dilatation was noted in some patients with dilated cardiomyopathy in the absence of epicardial vessel stenoses. These patients had a variable history of hypertension, but none were observed to be hypertensive at the time of perfusion imaging. There is limited coronary flow reserve as a possible explanation for transient ischemic dilatation of the left ventricle in patients with dilated cardiomyopathy. Patchy perfusion defects affecting the entire myocardium.
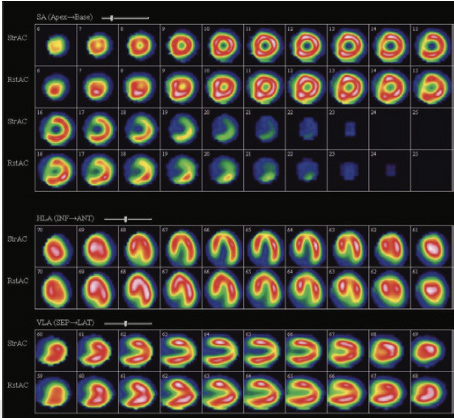
Figure 1
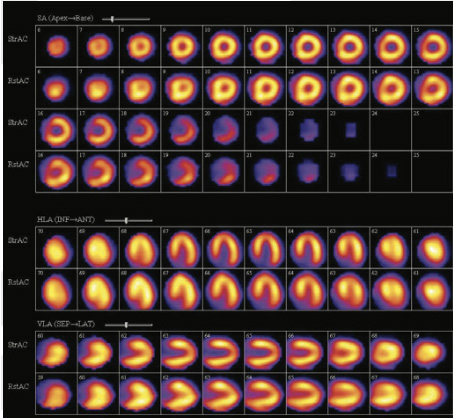
Figure 2

Figure 3
Mild reversible perfusion abnormality in apex, minimal reversible perfusion abnormality in septal wall close to apex, inferior wall close to apex and inferio-lateral wall of the myocardium (mid to base) of the myocardium at stress
The above mentioned perfusion abnormalities are not appreciated quantitatively and hence statistically NOT significant.