Meningiomas arise from the meningothelial cells of the arachnoid membranes, which are attached to the inner layer of the dura mater. They are the most frequently reported primary intracranial neoplasms representing about 20% of all intracranial tumors. They are usually diagnosed after the third decade of life, and they are more frequent in women than in men accounting for 38% of all intracranial tumors in women and 20% in men.
According to the World Health Organization (WHO) criteria, meningiomas can be classified into grade I meningiomas, which are benign, grade II (atypical) and grade III (anaplastic) meningiomas, which have a much more aggressive clinical behavior.
Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) are routinely used in the diagnostic workup of patients with meningiomas.
Molecular Nuclear Medicine Imaging with Hybrid SPECT-CT could provide complementary information to CT and MRI.
Various SPECT tracers may provide information about cellular processes and biological characteristics of meningiomas. Therefore, SPECT- CT imaging could be used for the preoperative noninvasive diagnosis and differential diagnosis of meningiomas, prediction of tumor grade and tumor recurrence, response to treatment, target volume delineation for radiation therapy planning, and distinction between residual or recurrent tumor from scar tissue.
Surgical resection of meningiomas is the prevalent treatment. Moreover, radiation therapy has now attained a standard role in the primary and adjuvant treatment settings and is indeed the only commonly accepted form of adjuvant therapy after surgical resection, although meningiomas have, errantly, been regarded as radio resistant. The risk of recurrence in operated meningiomas is correlated with the histological grade, the degree of surgical resection, the biological aggressiveness of the tumor, and large tumor size. Although in benign meningiomas surgical resection is associated with a high cure rate, supposing that the whole tumor is excised; however, there is always considerable risk of recurrence (ranged between 9% and 32%) even after apparently complete resection with excision of the surrounding dura and involved bone. On the contrary, recurrence is very common in high-grade lesions, even after total resection. Biological aggressiveness of meningiomas depends on various cellular characteristics such as the proliferation activity, which is determined by the expression of the nuclear antigen Ki-67.
Somatostatin—a cyclic tetra decapeptide neuropeptide—is the most widely distributed of the hypothalamic releasing hormones in the central nervous system and in the periphery, including the pancreas, gut, and pituitary. In the brain, somatostatin is believed to act as a neurotransmitter and neuromodulator.
The effects of somatostatin are mediated by transmembrane domain G-protein coupled receptors. In vivo and in vitro studies have shown that somatostatin receptors (SSTRs) are expressed on the cell membrane of various central nervous and peripheral tissues in high density to a varying extent, including tumors of neuroendocrine origin and intracranial tumors.
It is now well known that leptomeninx express SSTRs. Meningiomas, which originate from the arachnoid layer of the leptomeninx, usually have a high SSTR2 density.
Clinical nuclear medicine has taken advantage of this characteristic, making the in vivo detection of SSTRs possible. Somatostatin receptor scintigraphy (SSRS) became an invaluable tool used extensively in routine management of patients with meningiomas.
Although many studies have reported detection of all meningioma lesions with a sensitivity almost 100% Nevertheless, the method provides high negative predictive value (100%).
Due to the high SSTRs density, meningiomas of the skull base or the orbit may be differentiated from somatostatin receptor-negative tumors. Neurinomas and neurofibromas do not express somatostatin receptors, thus they do not demonstrate tracer uptake, making octreotide scintigraphy a useful technique in the differential diagnosis from multiple meningiomas.
SSRS seems to be an additional valuable tool with high sensitivity and specificity for optimizing the diagnosis of optic nerve sheath meningiomas from other orbital tumors (such as vascular lesions, non-hodgkin lymphomas, optic nerve gliomas, idiopathic orbital inflammation), without the need for more invasive procedures. It has been reported that the octreotide uptake ratios of meningiomas were significantly higher than those of other subgroups of orbital tumors, except for adenocarcinomas.
SSRS may also aid in the differentiation between postoperative scar and meningioma recurrence. The risk of local recurrence is well known, especially for meningioma located near the skull base where meninges are attached very closely to the bones, thus rendering total resection of meningioma difficult. In the first 6 months after surgery, in a reasonable number of patients, MRI may fail to differentiate between tumor remnants or recurrent meningioma and nonspecific hyper perfusion. The high tumor 111In-octreotide uptake seen in SSRS in most meningiomas may be of clinical relevance for exact tumor delineation in cases of tumor recurrence.
59 years old lady is operated case of cancer thyroid (PTC) with negative whole body iodine scan post operatively, but elevated thyroglobulin levels. Treated empirically with high dose of radioactive iodine therapy.
Had recurrence after four years in the cervical lymph nodes and biopsy showed recurrence with transformation to anaplastic thyroid carcinoma with lung metastasis. Treated than with again with radiation therapy, chemotherapy with no significant response. Shifted to immunotherapy with single agent Nivolumab for three years and achieved stable disease.
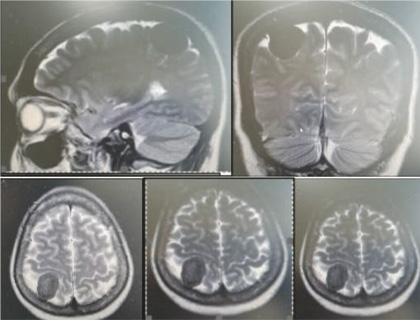
Most recent F18 FDG PET-CT showed progression of the pulmonary metastasis as well as cervical mediastinal lymphadenopathies. MRI BRAIN showed A well-defined intensity enhancing extra axial lesion in right posterior parietal region of the brain with erosion of the inner table, possibly representing meningioma.
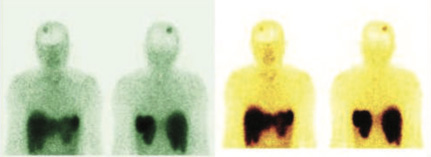
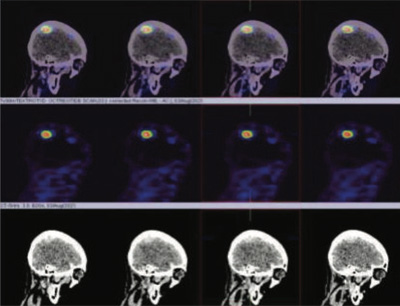
99mTc Tektrotyd labelled somatostatin receptor imaging was done to look for any other therapeutic option with Lutetium 177 DOTANEC, if the underlying cervical, mediastinal, and pulmonary metastasis shows positive SSR tracer uptakes.
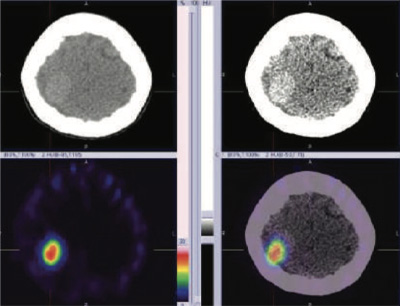
99mTc Tektrotyd (Octreotide) static and hybrid SPECT-CT images shows focal area of intense tracer uptakes (SRS expression lesion) in right posterior parietal
region of the brain consistent with MRI brain diagnosis of right posterior parietal region meningioma of the brain.
Rest of the whole-body images were unremarkable (No obvious abnormal tracer uptakes in cervical, mediastinal lymphadenopathy and pulmonary metastasis)
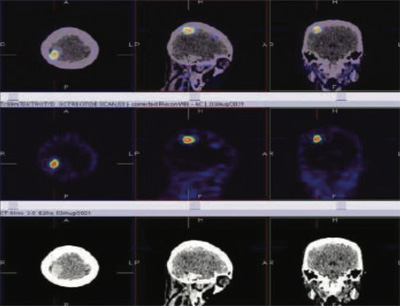
In the diagnostic workup of patients with meningiomas, a combination of contrast-enhanced imaging techniques such as Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) is routinely used in defining the location and extent of tumor, as well as for long-term follow-up. Although these imaging modalities could provide a presumptive diagnosis, the histological diagnosis can only be made postoperatively. Furthermore, this combination has also limitations, especially at the skull base and in the case of bony involvement, while in the case of suspected residual or recurrent tumor, it can be very difficult to distinguish viable tumor from scar tissue by CT or MRI.